The Spark That Split the Atom
A Discovery Tale about Energy, Curiosity, and the Power Within Everything
Journey through one of humanity's greatest scientific adventures—discovering what everything is made of and unlocking the incredible power hidden inside atoms.
The Invisible World
In a quiet laboratory in Cambridge, England, in 1897, a young scientist named J.J. Thomson stared at a mysterious glowing green tube. The room was dimly lit, and the only sound was the quiet hum of electrical equipment.

J.J. Thomson observing the cathode ray tube that revealed electrons
He wasn't studying ordinary light or simple electricity—he was trying to understand what matter itself was made of. What was the fundamental building block of everything in the universe?
Every rock, tree, drop of water, and person, he believed, was built from atomsThe smallest part of matter; everything is made of atoms—the smallest bits of the universe. The ancient Greeks had imagined atoms thousands of years earlier, but no one had ever seen one or proved they existed.
As Thomson ran electricity through his special tube—called a cathode ray tube—a strange greenish glow appeared. A beam shot from one end to the other, lighting up the glass. But here's what amazed him: when he moved a magnet nearby, the beam bent and curved!
"If light were just waves, a magnet wouldn't affect it this way," he reasoned. "There must be something smaller inside the atom—tiny particles that carry electric charge."
⚡ Thomson's Revolutionary Discovery:
He had just discovered the electronA tiny particle inside an atom that carries electric charge—the tiny particle with a negative charge that orbits the center of every atom. Electrons are incredibly small: it would take about 2,000 electrons to equal the mass of a single proton!
This discovery changed everything. It meant atoms weren't the smallest things after all—they had parts! It was like discovering that what you thought was a solid marble actually had an entire universe inside it.
Thomson proposed what became known as the "plum pudding model"—he imagined the atom like a pudding with electrons stuck in it like raisins. He was partly right about electrons, but the atom's structure was even stranger than he could imagine.
It was the first spark in a story that would change the world forever—and reveal a power hidden in every single atom.
Breaking the Indestructible
A few years later, in 1911, another brilliant scientist named Ernest Rutherford decided to test Thomson's plum pudding idea. If atoms were like soft pudding with electrons scattered throughout, then tiny particles should pass through them easily.
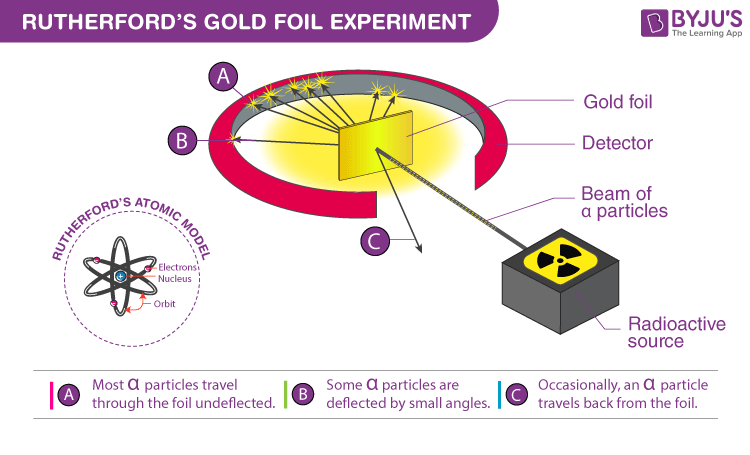
The shocking results of the gold foil experiment
Rutherford set up an elegant experiment. He took a very thin sheet of gold foil—so thin you could almost see through it, just a few atoms thick. Then he fired tiny alpha particles (bits of helium atoms) at it at tremendous speed.
According to Thomson's model, the alpha particles should pass straight through or maybe deflect slightly. Most did pass through without any problem, exactly as expected.
But then something astonishing happened: some of the alpha particles bounced straight back, as if they'd hit a brick wall!
"It's as if you fired a cannonball at tissue paper and it came back at you!" Rutherford exclaimed to his team, completely shocked. "It was quite the most incredible event that has ever happened to me in my life."
He realized that atoms weren't soft pudding at all. Instead, they were mostly empty space—like a huge stadium with a baseball at the center. But that tiny center, which he called the nucleusThe dense center of an atom that contains most of its mass, contained almost all of the atom's mass packed into an incredibly small, dense core.
🎯 How Empty Are Atoms?
Imagine if an atom were as big as a football stadium. The nucleus would be smaller than a marble sitting at the center of the field. The electrons would be tiny specs orbiting near the outer walls. Everything in between? Empty space!
This means YOU are mostly empty space. So is your chair, your house, and the ground beneath your feet. What makes things feel solid is the electromagnetic force between atoms pushing against each other—not because they're actually touching!
Rutherford later discovered that the nucleus itself contained positively charged particles he called protonsA positively charged particle inside the nucleus. Later, his student James Chadwick found another particle with no charge at all, which they named the neutronA particle inside the nucleus with no charge.
The atom had a heart—a tiny, powerful nucleus—and inside it, scientists would soon discover, was a storm of incredible power waiting to be unleashed.
The Hidden Energy
Years later, in the 1930s, a brilliant physicist named Lise Meitner was working in Berlin, Germany. She had dedicated her life to understanding the atomic nucleus—that mysterious, powerful center of the atom.
Lise and her research partner Otto Hahn studied atoms of uranium—one of the heaviest elements in nature. Uranium atoms have a huge nucleus packed with 92 protons and over 140 neutrons, making them unstable and reactive.

Lise Meitner, the physicist who understood nuclear fission
When they bombarded uranium with neutrons—shooting neutrons at uranium atoms like tiny bullets—something unexpected happened. The uranium nucleus didn't just absorb the neutron and get slightly bigger. Sometimes it split completely in half, breaking into two smaller atoms!
This was shocking. Atoms were supposed to be fundamental and unchangeable. But here they were, watching atoms split apart before their eyes.
Even more amazing: when the uranium atom split, it released an enormous burst of energyThe ability to make things move or change—far more energy than any chemical reaction could produce.
Lise calculated the numbers during a winter walk in Sweden (where she had fled to escape Nazi persecution). She and her nephew Otto Frisch realized what was happening: the uranium nucleus was like a wobbly water droplet. When hit by a neutron, it stretched and wiggled until it broke apart into two pieces.
They called this process fissionWhen an atom splits into smaller parts and releases energy—splitting the atom.
⚛️ Why Does Fission Release So Much Energy?
Einstein had discovered that mass and energy are related through his famous equation: E=mc². This means a tiny amount of mass can convert into a huge amount of energy (because c, the speed of light, is a very large number!).
When a uranium atom splits, the two resulting atoms together weigh slightly less than the original uranium atom. That "missing" mass? It converts directly into energy—heat, light, and motion.
Just one kilogram of uranium undergoing fission releases as much energy as burning 2.5 million kilograms of coal!
"So much energy from something so small," Lise whispered as she worked through the calculations. "It's the secret fire inside matter itself."
She realized immediately that this energy could power cities and change the world. But it could also be used for terrible weapons of destruction. The discovery filled her with both wonder and deep concern.
When World War II began and governments wanted to use her discovery to build atomic bombs, Lise Meitner refused. She believed that science should be used to help humanity, not harm it. She dedicated the rest of her life to peaceful research.
"I will have nothing to do with a bomb!" she declared firmly.
Power and Responsibility
In the decades that followed Meitner's discovery, scientists around the world grappled with what it meant. The same process could create miracles or catastrophes depending on how it was used.
Scientists built nuclear reactorsA machine that controls atomic energy to make electricity—carefully designed machines that use controlled fission to generate electricity. Inside a reactor, uranium atoms split one at a time in a carefully managed chain reaction. The heat from fission boils water, creates steam, and spins turbines to generate power.

A modern nuclear power plant producing clean electricity
One small fuel pellet of uranium—about the size of your fingertip—can produce as much energy as 150 gallons of oil or one ton of coal! A single nuclear power plant can provide electricity for hundreds of thousands of homes without producing air pollution or greenhouse gases.
Today, nuclear power provides about 10% of the world's electricity. In some countries like France, it's more than 70%. It helps fight climate change by providing large amounts of electricity without burning fossil fuels.
But the story has a darker chapter too. During World War II, other scientists used the same fission discovery to create atomic bombs—weapons of devastating power. When the first atomic bombs were used in 1945, entire cities were destroyed in an instant.
When Lise Meitner heard the news, she wept. Though she had refused to work on weapons, her scientific discovery had been used to create them anyway.
⚠️ The Weight of Discovery:
"We have opened a door," Lise said, "and now we must decide how to walk through it."
Her words remind us that scientific knowledge is neutral—it's neither good nor bad. What matters is how we choose to use it. Every major scientific discovery carries this same lesson: with great power comes great responsibilityThe duty to use knowledge or power wisely.
Scientists today continue to wrestle with these questions. How do we harness nuclear power safely? How do we prevent nuclear weapons from ever being used again? How do we dispose of radioactive waste responsibly?
These aren't just science questions—they're questions about what kind of world we want to create and what kind of future we want to build.
The Energy Within
Today, more than a century after Thomson discovered the electron, we know that atoms aren't the end of the story. In fact, they're just the beginning.
Inside the nucleus, protons and neutrons are themselves made of even smaller particles called quarks. Scientists use enormous machines called particle accelerators—some stretching for miles underground—to smash atoms together at nearly the speed of light, revealing the fundamental building blocks of reality.
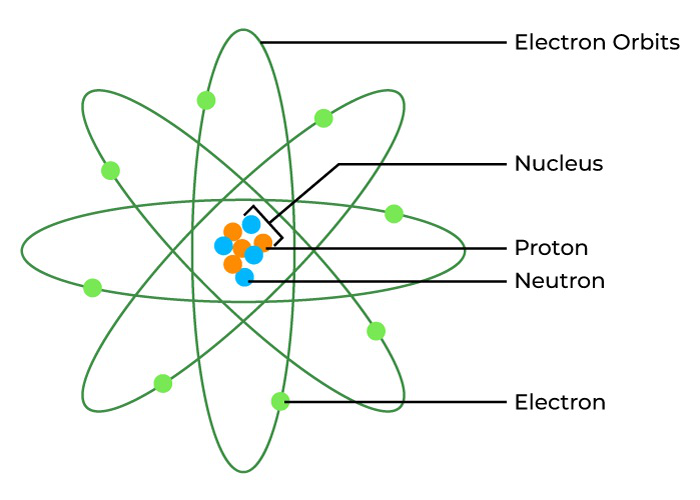
Our modern understanding of atoms—from electrons to quarks
But the same spark of curiosityThe desire to explore, learn, or understand that drove Thomson, Rutherford, and Meitner continues to light the path of discovery. Every time we ask "what if?" or "how does this work?" we're following in their footsteps.
And their discoveries touch your life every single day:
💡 Atoms in Your Daily Life:
- • Every phone, computer, and light bulb works because of electrons flowing through circuits—Thomson's discovery
- • MRI machines in hospitals use atomic nuclei to create detailed images of the inside of your body
- • Smoke detectors contain tiny amounts of radioactive material that detect smoke particles
- • The sun itself is powered by nuclear fusion—the opposite of fission, where atoms combine instead of split
- • Carbon dating uses radioactive atoms to tell us how old fossils and artifacts are
- • GPS satellites need to account for atomic physics to give you accurate directions!
When you turn on a light switch, electrons flow through wires. When you charge a battery, you're storing energy by moving electrons around. When you see colors, different atoms in objects are absorbing and reflecting light at different wavelengths. Everything you touch, see, and experience is atoms interacting with atoms.
Scientists are now working on nuclear fusion—the process that powers the sun—hoping to create a nearly limitless source of clean energy here on Earth. Fusion would combine small atoms like hydrogen into helium, releasing energy without creating dangerous waste or the risk of meltdowns.
"There is more energy in a grain of sand," Lise Meitner once said, "than anyone can imagine."
And it all began with one scientist who dared to ask: What's inside the invisible? What happens when we look deeper than anyone has looked before?
🌟 The Legacy of Atomic Discovery:
Thomson, Rutherford, and Meitner showed us that the universe is more wonderful and strange than we ever imagined. They taught us that even the smallest things contain incredible power and beauty.
But they also taught us that knowledge brings responsibility. Every discovery is a choice: will we use it to build or to destroy? To help or to harm? To illuminate or to darken?
The next great discoveries are waiting. Maybe you'll be the one to unlock fusion energy. Maybe you'll discover new particles. Maybe you'll find ways to use atomic power that we haven't even imagined yet.
All it takes is curiosity, careful thinking, and the courage to look where no one has looked before.
The invisible world is waiting. What will you discover?
🧠 Science Vocabulary
Atom
The smallest part of matter; everything is made of atoms.
Electron
A tiny particle inside an atom that carries electric charge.
Nucleus
The dense center of an atom that contains most of its mass.
Proton
A positively charged particle inside the nucleus.
Neutron
A particle inside the nucleus with no charge.
Fission
When an atom splits into smaller parts and releases energy.
Energy
The ability to make things move or change.
Nuclear Reactor
A machine that controls atomic energy to make electricity.
Responsibility
The duty to use knowledge or power wisely.
Curiosity
The desire to explore, learn, or understand.
🔬 Try It Yourself: Chain Reaction Demonstration
Model a Nuclear Chain Reaction
See how one event can trigger many others, just like neutrons causing atoms to split!
Materials Needed:
- 20-30 dominoes
- Flat surface
- Timer or stopwatch (optional)
- Camera to record (optional)
Instructions:
- Stand dominoes in a line, close enough that each will knock down the next
- Gently push the first domino
- Watch as each falling domino knocks down the next
- Try different patterns: straight lines, curves, branches splitting into two paths
- Experiment with spacing—what happens if dominoes are too far apart?
🤔 Think About It:
- • How is this like nuclear fission? (One neutron hits a uranium atom, causing it to split and release more neutrons, which hit more atoms...)
- • What happens if one domino is missing? (Like control rods in a reactor that absorb neutrons to slow the reaction)
- • What if you have two lines branching? (Each uranium atom releases 2-3 neutrons, multiplying the reaction)
- • How could you "control" your domino chain reaction to make it slower or stop it?
Extension Activity: Build an Atom Model
Create a 3D model of an atom:
- Use a large ball or styrofoam sphere for the nucleus
- Add small beads or balls: red for protons (+), blue for neutrons (neutral)
- Use pipe cleaners or wire to create electron orbits
- Add tiny beads for electrons (-) on the orbits
- Try making different elements: Hydrogen (1 proton, 1 electron), Helium (2 protons, 2 neutrons, 2 electrons), Carbon (6 of each)
- Remember: the real atom would be mostly empty space!
💬 Discussion Questions
1. What did scientists discover when they looked inside the atom?
Think about: What did Thomson find? What surprised Rutherford? What did Meitner realize about uranium? How did each discovery change our understanding?
2. How did splitting atoms change the world—for good and for bad?
Consider: What are the benefits of nuclear power? What are the dangers? Why did Lise Meitner refuse to work on weapons?
3. What does it mean to use science responsibly?
Reflect: Can scientific knowledge be "good" or "bad"? Who decides how discoveries are used? What responsibilities do scientists have? What about the rest of us?
4. Why do you think curiosity is important for discovery?
Explore: What questions drove these scientists? What did they wonder about? How can asking "what if?" lead to world-changing discoveries?